Volume I, Section 7
7 HEALTH MANAGEMENT
{OP} For a description of the notations, see Acceleration
Regimes.
This section contains the following topics: 
7.1 Introduction
7.2 Preventive Care
7.3 Medical Care
7.4 Crew Survival
See the video clips
associated with this section.
7.1
INTRODUCTION
{A}
This section discusses the measures that must be taken to maintain
the health of the crew. The following topics are covered:
a. Preventive Care - Non-medical measures that must be taken to preserve
crew health.
b. Medical Care - Medical functions of prevention, diagnosis, and treatment.
This section discusses only functional considerations and requirements.
Sections that discuss facilities and equipment required to implement
these functions are referenced in applicable paragraphs.
7.2
PREVENTIVE CARE
{A}
7.2.1 Introduction
{A}
This section identifies and discusses the activities considered necessary
for a crewmember to maintain good health in a reduced gravity environment.
The subsections discuss the consideration and requirements for the following
preventive care measures:
a. Nutrition.
b. Reduced gravity countermeasures.
c. Health monitoring.
d. Sleep.
e. Personal hygiene.
f. Pre- and post-mission health management.
Facilities and equipment for implementation of preventive care are
discussed in Section 10.0, Activities Centers.
7.2.2 Nutrition
{A}
7.2.2.1 Introduction
{A}
This section discusses the food and water intake requirements of the
crewmembers. The information applies primarily to an IVA environment
and reduced gravity conditions. The water requirements apply to potable
water only.
(Refer to Paragraph 7.2.5.3.6, Personal Hygiene
Water Requirements, for information on water for personal hygiene.)
(Refer to Paragraph 14.2.3.6,
EVA Food and Drinking Water Design Requirements, for specific nutritional
requirements when performing EVA activities.)
7.2.2.2 Nutrition
Design Considerations
{A}
7.2.2.2.1 Goal
of Nutrition Program Design Considerations
{A}
The goal of the nutrition program is to establish an Earth-normal pattern
and quality of meals while meeting the physiological requirements of
the crew.
7.2.2.2.2 Food
Acceptability Design Considerations
{A}
The following factors affect the acceptability of the food and the
appetite of the crewmembers:
a. Past Experience And Personal Preference - Generally, a taste for
new foods must be acquired. This will be an important consideration
with international crews. Pre-mission crew selection of menus is desirable.
b. Variety - Food can lose its acceptance if eaten too frequently.
A wide variety of foods is desirable. Food may also be varied by changing
the form, texture, and flavor, without affecting nutritional content.
The use of colors, shapes, garnishes, and portions in meal presentation,
as well as packaging color, utensil shape and size, and visual display
of trays may also enhance the eating experience.
c. Waste Management Facilities - In the past, inadequate body waste
management facilities have discouraged food consumption.
(Refer to Paragraph 10.3,
Body Waste Management, for design requirements of body waste management
facilities.)
d. Space Adaptation Syndrome - Control of Space Adaptation Syndrome
is essential for a better appetite.
(Refer to Paragraph 7.2.3.4.3, Nonexercise
Countermeasures Design Requirements, for additional information.)
e. Atmospheric Contaminants - The buildup of background odors during
missions may contribute subliminally to a decrease in appetite and consumption
as a result of fatigue or adaptation.
(Refer to Paragraph 5.1,
Atmospheric Control, for additional information.)
f. Availability - Snacks should be available with a minimum of preparation.
This is particularly important for high energy output tasks such as
EVA operations.
g. Food Form - The more Earth-normal quality of the food, the more
acceptable it will be. This includes the desirability of fresh fruits
and vegetables. Precooked frozen food has the highest overall acceptability
of the current available methods of preservation.
h. Meal Scheduling - Lack of consistent meal periods in the crew schedule
can lead to skipped meals and undernourishment.
i. Microgravity Environment - Some U.S. and Soviet space crews have
reported that changes in taste and odor perception of foods occur during
space flights. This may be due to body fluid shift and resulting head
congestion.
(Refer to Paragraph 4.4,
Olfaction and Taste, for additional information.)
7.2.2.2.3 Food
and Water Quality and Quantity Design Considerations
{A}
The type and quantity of food and liquid required by an individual
is dependent on a number of factors. These factors must be considered
when establishing an individual menu. These factors include:
a. Crewmember Size and Activity Level - The level of activities and
size of the crewmember influence the required calories and water intake.
EVA activities, for instance, require a higher energy output.
b. Microgravity Effects - There are many unknown factors involved in
the area of microgravity space nutrition and metabolism. The food provided
must be varied and easily accessible such that a crewmember's individual
needs and cravings can be satisfied. The food provided must be of sufficient
quality, quantity, and nutrient content to meet the energy demands of
various activities (e.g., EVA, countermeasure training, daily work activities),
while accommodating each crew member's individual needs and desires.
c. Food Rehydration - The total amount of potable water required depends
in part on the food rehydration requirements of the mission. This is
illustrated in Figure 7.2.2.2.3-1,
which shows food and water quantity requirements for varying levels
of food hydration. The total water per person-day (rehydration water,
drinking water, and water in food and beverages) is assumed to be 3
Kg (6.6 lbs). In the Figure, food packaging is assumed to be 0.5 Kg
(1.1 lbs) and dry food weight 0.7 Kg (1.6 lbs).
d. Space Module Environment - Space module temperature and humidity
impact the amount of water ingested.
Figure
7.2.2.2.3-1 Typical Mass of Food and Water per Person Day for Varying
Levels of Food and Beverage Hydration
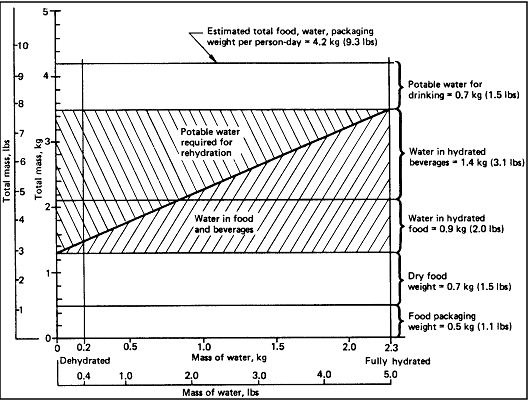
Reference: 107, p. 410;
NASA-STD-3000 185
7.2.2.3 Nutrition
Design Requirements
{A}
7.2.2.3.1 Food
Design Requirements
{A}
The food provided shall meet the following requirements:
a. Minimum Nutritional Requirements.
1. The diet shall supply the nutritional quality required by JSC 32283
(Nutritional Requirements for Extended Duration Missions .
2. Additional nutritional requirements are required for EVA as per
body size, EVA tasks, and duration of EVA. Foods and fluids shall be
specifically allocated for this requirement per
Paragraph 14.2.3.3.
b. Nutritional Program Monitoring - An automated nutrient monitoring
process shall be provided that meets the nutrient monitoring requirements
as specified in JSC 32283. Parameters required for medical or investigative
purposes shall include documenting:
1. the crewmember that consumed the food.
2. amount of food consumed
3. time and date of consumption,
4. food item/lot number/serial number.
Data shall be downlinked periodically for analysis and must provide
information in format acceptable for post flight analysis.
c. Microbiology - Microbiological acceptability limits shall be as
given in Figure 7.2.2.3.1-1.
Figure
7.2.2.3.1-1Microbiology Contamination Control Specification For
Crew Food
| AREA/ITEM |
MICROORGANISM TOLERANCES |
| 1. Food Production Area |
Samples Collected* |
Limits (CFU**) |
| a. Surfaces |
3 surfaces sampled per day |
<=3/cm2 |
| b. Packaging Film |
Before use |
<=3/cm2 |
| c. Food Processing Equipment |
2 pieces sampled per day |
<=3/cm2 |
| d. Air |
1 sample of 0.282 M3 (10 ft) |
<=13/320 liters |
| 1. Food Production Area |
Samples Collected* |
Limits (CFU**) |
| a. Nonthermostabilized |
Total aerobic count |
<=10,000/g |
|
Escherichia coli |
<=1/g |
|
Coagulase postitive Staphylocci |
<=1/5g |
|
Salmonella |
<=1/25g |
|
Clostridium Perfringens |
<100g |
|
Yeast and molds |
<100/g |
| Notes:
* Sample collected only on days that food facility is in operation
** Total aerobic count |
Reference: 406; NASA-STD-3000
508
7.2.2.3.2 Potable
Water Design Requirements
{A}
a. Quality - The potable water quality requirements are given in
Figure 7.2.2.3.2-1
b. Quantity - The supply of available water for drinking and hydration
of food dependent on degree of food hydration shall be given in
Figure 7.2.2.3.2-2, the potable water quantity requirements. The
supply of available water for drinking and rehydration of food is listed
below:
1. Operational Mode - 2.84 to 5.16 Kg per person-day (6.26 to 11.35
lbs per person-day)
2. Degraded and Emergency Mode - 2.84 Kg per person-day (6.26 lbs per
person-day)
c. Emergency - The supply of available water for drinking and rehydration
of food shall be a minimum of 0.95 Kg (2.1 lbs) per person for each
eight hours of anticipated emergency vehicle occupancy time, including
orbital loiter time and time on the earth's surface without rescue services.
An additional 1 Kg (2.2 lbs) of water and 8 one gram salt tablets shall
be provided for each person for the purpose of supporting reentry fluid
loss countermeasures.
d. Temperature - Drinking water temperatures shall be as follows:
1. Cold Water - Cold water temperature shall be 1.6 degrees to 7.2
degrees C (35 degrees to 45 degrees F).
2. Ambient Water - Ambient water temperature shall be 15.5 degrees
to 26.7 degrees C (60 degrees to 80 degrees F).
3. Hot Water - Means shall be provided for heating water up to 68 degrees
± 2.8 degrees C (155 degrees ± 5 degrees F).
Figure
7.2.2.3.2-1 Potable Water Quality Requirements (Maximum Contaminant
Levels)
| QUALITY PARAMETERS |
LIMITS |
| PHYSICAL PARAMETERS |
|
| Total solids (mg/l) |
100 |
| Color True (Pt/Co units) |
15 |
| Taste (TTN) |
3 |
| Odor (TON) |
3 |
| Particulates (max size - microns) |
40 |
| pH |
6.0 - 8.5 |
| Turbidity (NTU) |
1 |
| Dissolved Gas (free @ 37°C) |
Note 1 |
| Free GAss (@ STP) |
Note 1 |
| INORGANIC CONSTITUENTS (mg/l) (see Notes 2 and 5) |
|
| Ammonia |
0.5 |
| Arsenic |
0.01 |
| Barium |
1.0 |
| Cadmium |
0.005 |
| Calcium |
30 |
| Chlorine (Total - Includes Chloride) |
200 |
| Chromium |
0.05 |
| Copper |
1.0 |
| Iodine (Total - Includes Organic Iodine) |
15 |
| Iron |
0.3 |
| Lead |
0.05 |
| Magnesium |
50 |
| Manganese |
0.05 |
| Mercury |
0.002 |
| Nickel |
0.05 |
| Nitrate (NO3-N) |
10 |
| Potassium |
340 |
| Selenium |
0.01 |
| Silver |
0.05 |
| Sulfate |
250 |
| Sulfide |
0.05 |
| Zinc |
5.0 |
| BACTERICIDE (mg/l) |
|
| Residual Iodine (minimum) |
0.5 |
| Residual Iodine (maximum) |
4.0 |
| AESTHETICS (MG/L) |
|
| Cations |
30 |
| Anions |
30 |
| CO2 |
25 |
| MICROBIAL |
|
| Bacteria (CFU/100 ml) |
|
| Total Count |
1 |
| Anaerobes |
1 |
| Coliform |
1 |
| Virus (PFU/100 ml) |
1 |
| Yeast & Mold (CFU/100 ml) |
1 |
| RADIOACTIVE CONSTITUENTS (pCi/l) |
Note 3 |
| ORGANIC PARAMETERS (µµg/l) (see Note 2) |
|
| Total Acids |
500 |
| Cyanide |
200 |
| Halogenated Hydrocarbons |
10 |
| Total Phenols |
1 |
| Total Alcohols |
500 |
| Total Organic Carbon (TOC) |
500 |
| Uncharacterized TOC (UTOC) (µg/l) (see
Note 4) |
100 |
| ORGANIC CONSTITUENTS (mg/l) (see Notes 2 & 5) |
|
| Notes:
1. No detectable gas using a volumetric gas vs. fluid measurement
system. Excludes CO2 used for aesthetic purpose.
2. Each parameter/constituent MCL must be considered individually
and independency of others.
3. The maximum contaminant level for radioactive constituents
in portable and personal hygiene water shall conform to Nuclear
Regulatory Commission (NRC) regulations (10CFR20, et al.). These
maximum contaminant levels are listed in the Federal Registry,
Vol. 51, No. 6, 1986, Appendix B, as Table 2 (Reference Level
Concentrations) Column 2 (Water). Control/containment/monitoring
of radioactive constituents shall be the responsibility of the
user. Prior to the introduction of any radioactive constituents
shall be obtained from the Radiation Constraints Panel (RCP).
The RCP will approve or disapprove proposed monitoring and decontamination
procedures on a case-by-case basis.
4. UTOC equals TOC minus the sum of analyzed organic constituents,
expressed in equivalent TOC.
5. In the event a quality parameter not listed in this table
is projected, or found, to be present in the reclaimed water,
the Water Quality Manager from NASA authorizing authority shall
be contacted for a determination of the MCL for that parameter. |
NASA-STD-3000 418
Figure
7.2.2.3.2-2 Potable Water Quantity Requirements
| UNITS |
MODE |
| Operational |
90 Day Degraded1 |
Emergency3 |
| lb/person-day |
6.262 - 11.352 |
6.262 |
6.262 |
| kg/person-day |
2.84 - 5.16 |
2.84 |
2.84 |
| Notes:
1 Degraded levels meet "fail operational criteria".
2 Based on 2950 kcal/person-day IVA work rate. Actual amount
depends on degree of hydration of the food.
3 Safe Haven conditions shall be maintainable for up to 45 days. |
Reference: 278 With Updates;
NASA-STD-3000 409, Rev. A
7.2.2.4 Example Nutritional Program
{O}
The menus on the Space Transportation System (Shuttle) are designed
to provide the nutrients in Figure 7.2.2.4-1
in three meals per person per day.
Figure
7.2.2.4-1 Example Nutrition Program
Nutrient Requirements in Three Meals Per Person Per Day
| Energy (kcal) |
WHO equation |
Vitamin B6 (mg) |
2.0 men 1.6 women |
| Protein % of calories |
12 - 15 |
Vitamin B12 (µg) |
2.0 |
| Vitamin A (RE)
|
1000 Ng/d men
800 Ng/d women |
Calcium (mg) |
800 - 1200 |
|
10 |
Phosphorus (mg) |
800 - 1200 |
| Vitamin E (TE) |
10 men
8 women |
Iodine (mg) |
150 |
| Ascorbic acid (mg) |
100 |
Iron (mg) |
10 |
| Folate (µg) |
200 men
180 women |
Magnesium (mg) |
350 men
280 women |
| Niacin (mg NE) |
19 men
15 women |
Zinc (mg) |
15 men
12 women |
| Riboflavin (mg) |
1.7 men
1.3 women |
Potassium (mg) |
3500 |
| Thiamin |
1.5 men
1.1 women |
Sodium (mg) |
1100 - 3300 |
Reference: 309, p. 23
NASA-STD-3000 - 157, 406 With Updates
Figure
7.2.3.1-1 Time Course of Physiological Shifts Associated with Acclimation
to the Micro-g Environment.
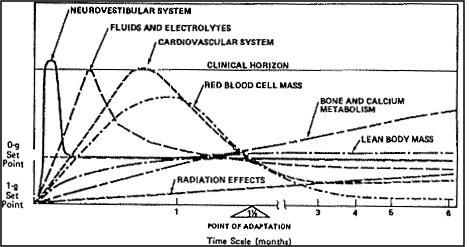
Reference: 208, Figure
1, p. 134; NASA-STD-3000 408
7.2.3 Reduced Gravity
Countermeasures
{OP}
7.2.3.1 Introduction
{OP}
The following section discusses the effects of gravitational changes
on the human body and countermeasures that can be taken to maintain
crew health. Space gravity environments can vary from multi-G (during
launch) to microgravity in orbit. This section primarily deals with
microgravity. The considerations and requirements apply to all reduced
gravity conditions but proportionally less with increasing gravity.
Specific countermeasure requirements for reduced gravity greater than
microgravity must be established on an individual basis.
Figure 7.2.3.1-1 illustrates the time course of shifts in various
physiological parameters associated with acclimation to the micro-g
environment.
7.2.3.2 Reduced Gravity Countermeasures Design Considerations
{OP}
Reduced gravity countermeasures fall into three general categories
which are described below:
a. Countermeasures Against Initial Response to Reduced Gravity - There
are several responses that begin within the first few hours of exposure
to reduced gravity and continue for from one to five days depending
on the individual. These responses are:
1. Vestibular side effects and space motion sickness.
2. Reduced motor skills due to unfamiliarity with reduced gravity.
3. Loss of Body Fluids - Body fluids shift headward in reduced gravity
conditions, resulting in increased urination and fluid loss.
(Refer to Paragraph 7.2.3.4, Nonexercise Countermeasures,
for information on countermeasures for the above effects.)
(Refer to Section 4.0, Human Performance
Capabilities, for information on vestibular effects and motor performance
in reduced gravity.)
b. Maintenance of 1-G Conditioning - The body slowly loses conditioning
with exposure to reduced gravity. Counter
measures should be considered against at least the following three
deconditioning effects:
1. Loss of strength and muscle mass.
2. Loss of bone minerals.
3. Loss of cardiovascular conditioning.
Exercise is the primary countermeasure against these effects and is
discussed in Paragraph 7.2.3.3.
c. Countermeasures Against Initial Response Upon Entry to 1-G - After
exposure to reduced gravity, the human body makes several immediate
adjustments when exposed to 1-G conditions.
The following are responses for which countermeasures should be considered:
1. Dehydration - This is due to the fluid losses during microgravity.
2. Rapid shift of fluids to the lower body due to re-exposure to the
1-G environment.
3. Reduced motor capabilities in 1-G.
Countermeasures against these effects are discussed in
Paragraph 7.2.3.4.
7.2.3.3 Exercise Countermeasures
{OP}
7.2.3.3.1 Introduction
{OP}
This section discusses the exercise countermeasures against the deconditioning
effects of reduced gravity that have resulted in loss of muscular strength
and cardio-respiratory endurance.
(Refer to
Paragraph 10.8, Microgravity Countermeasures, for information on
the facility requirements for these exercise countermeasures.)
7.2.3.3.2 Exercise Countermeasures Design Considerations
{OP}
7.2.3.3.2.1 Deconditioning Effects Of Reduced G Design Considerations
{OP}
Two of the most immediate and significant effects of microgravity are
the removal of weight forces from bone and muscle, and the headward
shift of fluids. These changes lead to a progressive degradation of
muscles, the skeletal system, and cardiovascular conditioning by Earth's
standards. Musculoskeletal system changes, brought about by lack of
exercise and the absence of gravitational forces, are mostly reversible,
but they contribute to weakness and poor gravitational tolerance in
the post-mission period. Cardiovascular deconditioning is manifested
by a post-mission orthostatic intolerance, decreased cardiac output,
and reduced exercise capacity. Both forms of deconditioning may impair
the ability of an individual adapted to weightlessness to function and
perform adequately during EVA or the critical phases during entry and
landing.
7.2.3.3.2.2 Deconditioning Countermeasure Design Considerations
{OP}
Because the underlying factor producing the changes leading to both
cardiovascular and musculoskeletal deconditioning in the absence of
gravity, the effort to reduce these deconditioning effects has been
primarily focused on restoring weight forces, stresses, and system interactions
by simulating Earth-normal physical movements. The single approach which
so far has received wide operational acceptance in the U.S. and USSR
space programs is exercise. The following are considerations to be made
when designing a reduced gravity exercise countermeasure program:
a. Type of Exercise - Exercises necessary to counteract the effects
of reduced gravity are listed in Figure
7.2.3.3.2.2-1.
b. Mission Duration - For short-term missions (less than 10 days),
pre-mission conditioning of crewmembers to elevated levels of fitness
should compensate for the anticipated decrements in physiological function
so that impairment during entry, landing and post-mission will be tolerable.
Even for these short missions, however, in the interest of crew morale,
some opportunity to exercise should be provided. For missions during
which crewmembers will be exposed to microgravity for greater than 10
days, deconditioning countermeasures will be essential.
c. Limitations of Exercise Program - Until recently, it was believed
that a proper exercise program could reverse the significant physiological/anatomical
changes associated with the body's response to microgravity. However,
studies of prolonged bed rest suggest that exercise, by itself, is insufficient
to meet these ends. For instance, changes in endocrine and metabolic
functions now are believed to result from changes in hydrostatic pressure
and from lack of postural cues, rather than from a lack of activity.
There is a possibility, however, that activities of higher intensity
or longer duration could have countered these changes.
d. Motivational Factors - Motivation is an extremely important consideration.
All of the planning and equipment can be wasted if the crewmembers are
not motivated to participate in an exercise program. The following factors
play an important role in the motivation of crewmembers:
1. Understanding - The crewmembers should be aware that the exercise
program is providing positive benefits to their health and will benefit
both in a reduced gravity environment and in their eventual return to
Earth. For protracted durations in space, exercise countermeasures may
be essential to mission fulfillment.
2. Feedback - Performance monitoring/display devices and record keeping
to evaluate progress are known tools for enhancing motivation. Video
displays and computerized programs are available for exercise equipment
in a myriad of formats. This approach can be utilized to provide this
feedback, as well as entertain the exercising crewmember.
3. Entertainment and Diversion - Video, music, reading materials, social
interaction, Earth-viewing windows, etc., may act as a diversion to
keep exercise from becoming monotonous for some crewmembers.
4. Games - Designing exercises that have an element of play can provide
positive motivation. For instance, gravity forces less than 1-G constitute
a new environment with a new set of physical challenges.
5. Facilities - Facilities that require little preparation prior to
exercise and minimal stowage afterward are essential if crewmembers
are to maintain motivation and adherence to the program.
(Refer to
Paragraph 10.8, Microgravity Countermeasures, for information on
exercise facility design.)
6. Support Facilities - Adequate facilities for washing and resting
after exercise are necessary for motivation.
(Refer to Paragraph
10.2, Personal Hygiene, for information on body washing facility
design and to Paragraph
10.4, Crew Quarters, for information on resting facility design.)
Figure
7.2.3.3.2.2-1 Exercise Countermeasure Design Considerations
| Problem |
Exercise |
Comment |
| Bone mineral loss |
Impact activities plus maximum isometric strength exercises |
While evidence is mixed, these attempts should be continued;
treadmill is suitable for impact activity |
| Muscular strength losses |
Low frequency, high resistance exercises for all major muscle
groups |
Simulated one-g weight training program can be used, for instance,
a rowing action ergometer. |
| Cardiovascular function (aerobic power) loss |
Aerobic type exercise |
Cycle and rowing type ergometer superior to treadmill for
monitoring, quantifying, and ease of use. |
NASA-STD-3000 189
7.2.3.3.3 Exercise
Countermeasure Design Requirements
{OP}
Exercise countermeasure requirements apply to space missions that expose
crewmembers to microgravity conditions for longer than 10 days. For
missions of 10 days or less, exercise countermeasures shall be available
for crewmembers as necessary to maintain performance during the mission
on entry to 1-G. The following are the exercise countermeasure design
requirements:
a. Types of Exercise - The space module shall provide facilities for
the following types of exercise:
1. Equipment for placing isokinetic, isotonic (concentric and eccentric),
and isometric force upon the major muscle groups of the body shall be
provided in order to counter disuse atrophy caused by microgravity.
2. Devices for exercising the cardiorespiratory system by engaging
large skeletal muscle masses (i.e., aerobic exercises) as partial countermeasure
to cardiovascular deconditioning shall be provided.
b. Duration of Exercise - Facilities and scheduling shall provide the
capability for all crewmembers to exercise not less than 1 hour per
day.
c. Exercise Regimens - Capability shall be provided for establishing
and updating individualized exercise routines and goals for each crewmember.
d. Motivation and Training - Appropriate motivational devices and/or
incentives shall be provided. Crewmembers shall be trained in the importance
of exercise and how to use the equipment.
e. Data Monitoring of Exercise - There shall be the capability to monitor
physiological parameters during exercise, store the data, and downlink
this information to Earth. The following physiological parameters shall
be monitored:
1. Routine Monitoring.
(a) Heart Rate.
(b) Duration of Exercise Period
(c) Power Output From Instrumented Exercise Device
2. Periodic Monitoring
(a) Electrocardiogram
(b) Blood Pressure
(c) Maximal and Submaximal Oxygen Uptake
(d) Muscle Performance
(e) Body Mass Measurement
7.2.3.4 Nonexercise
Countermeasures
{OP}
7.2.3.4.1 Introduction
{OP}
This section discusses the nonexercise countermeasures for the deconditioning
effects of reduced gravity. Nutrition, which plays a supportive role
to other countermeasures, is discussed in Paragraph
7.2.2.
7.2.3.4.2 Nonexercise Countermeasures Design Considerations
{OP}
Exercise is the primary countermeasure against the body deconditioning
effects of extended exposure to reduced gravity conditions. Other body
effects appear more rapidly upon changes in the gravity environment.
These effects should also be considered in mission planning and in providing
countermeasures. The significant immediate effects due to changes in
gravity conditions are as follows:
a. Vestibular side effects and space motion sickness in reduced gravity.
b. Loss of body fluids soon after exposure to reduced gravity (this
results in dehydration when the fluids redistribute on exposure to 1-G
conditions).
c. Reduced motor performance in a novel gravity environment (this requires
training and adaptation and occurs both on entry to 1-G and microgravity
conditions).
7.2.3.4.3 Nonexercise
Countermeasures Design Requirements
{OP}
Nonexercise countermeasures shall be provided regardless of the duration
of the mission. The following are required countermeasures:
a. Pressurized Countermeasures - Lower body positive pressure devices
for gravity protection during 1-G entry and landing shall be provided.
b. Pharmacological Countermeasures - Pharmacological methods, including
oral rehydration, shall be provided to increase the body's total fluid
volume. These countermeasures shall be available for implementation
just prior to entry into a 1-G environment.
c. Space Motion Sickness Countermeasures - Space motion sickness countermeasures
shall be provided and shall include:
1. Prophylactic medication.
2. Scheduling so that activities which require head and body translation
movements are minimized during the early days of the mission.
{A}
7.2.4.1 Introduction
{A}
This section on sleep includes:
a. Effects of microgravity on sleep needs.
b. Scheduling.
c. Duration.
d. Sleep aids.
(Refer to Paragraph 10.4,
Crew Quarters, for information on facilities to support sleep.)
7.2.4.2 Sleep Design Considerations
{A}
The following are considerations to be made when establishing a space
module sleep schedule and facility in a microgravity environment.
a. Effects of Microgravity - The results of Skylab experiments do not
show any major adverse changes in sleep as a result of prolonged space
flight. Only during the 84 day flight did one subject experience any
real difficulty in terms of sleep time. Even then, the problem diminished
with time, although sleeping medication was required on occasion. The
most significant changes occurred in the postflight period, with alterations
more of sleep quality than quantity. It appears that readaptation to
a 1-G environment is more disruptive to sleep than the adaptation to
microgravity. In all, the Skylab investigators feel that adequate sleep
can be obtained in a microgravity environment providing adequate sleeping
areas are used, noise levels are minimized, and a familiar time reference
for the sleep period is used.
b. Duration of Sleep - Satisfactory psychological performance is dependent
upon an adequate sleep/wakefulness cycle, but few studies have been
done to determine the optimum number of hours of sleep required per
hours of waking time. The usual study has investigated the amount of
sleep spontaneously taken per day without regard to performance. It
has not been demonstrated at this point whether humans need 6 to 8 hours
of sleep in every 24. On the short side, the quality of afternoon performance
improves almost linearly as sleep duration is increased from 1 to 6
hours. Beyond a duration of 6 hours of sleep, improvement is less marked
and is completely absent when sleep is lengthened from 8 to 10 hours
in every 24.
c. Sleep/Work Cycle - The following factors must be considered about
sleep/work cycles:
1. Personnel exposed to changes in environmental cues will show disrupted
circadian rhythms.
2. Circadian rhythms significantly affect a wide variety of human functions
in addition to sleep, including psychomotor and cognitive performance,
mood, and social adaptability.
3. Careful planning of activity schedules, sleep/wake schedules, and
artificial control of environmental cues may be necessary to offset
the possible negative impact of circadian desynchronization on crew
performance and adjustment.
4. Sleep periods should be proceeded by at least 1 hour of nondemanding
mental activity.
7.2.4.3 Sleep Design Requirements
{A}
The following are design requirements for crew sleep:
a. Facilities - Adequate sleep facilities shall be provided.
(Refer to Paragraph 10.4,
Crew Quarters, for sleep facility design requirements.)
b. Duration - Scheduling should allow a minimum sleep period of 8 hours
per day with minimum of 6 hours of uninterrupted sleep.
c. Pharmaceuticals - Appropriate sleep aid medication shall be made
available to crewmembers via a controlled access system.
7.2.5 Personal Hygiene
{A}
7.2.5.1 Introduction
{A}
This section on personal hygiene includes the functional considerations
and requirements for maintaining proper personal hygiene during a space
mission.
(Refer to Paragraph
10.2, Personal Hygiene, for information on facilities supporting
personal hygiene.)
7.2.5.2 Personal Hygiene Design Considerations
{A}
Personal hygiene is important to both the psychological and physiological
well being of the crew. The following are considerations for ensuring
a proper personal hygiene program:
a. Facilities - Facilities for performing personal hygiene functions
must be properly sized and accessible.
b. Equipment, Supplies, and Clothing - Personal hygiene equipment and
supplies and crew clothing must accommodate the physiological differences
in male and female crew members in the microgravity environment. The
hardware should make this accommodation with as few interchangeable
components as possible. The supplies and clothing should also be able
to meet the personal tastes and needs of the crew members to the extent
possible in the space module environment.
c. Training - Crewmembers must be adequately trained and familiar with
both personal hygiene equipment and procedures.
d. Scheduling - Proper scheduling must be provided to allow adequate
time for personal hygiene.
e. Personal Hygiene Standards - Personal hygiene standards should be
established prior to the start of the program.
7.2.5.3 Personal Hygiene Design Requirements
{A}
7.2.5.3.1 Body Grooming Design Requirements
{A}
The following body grooming measures shall be provided in the space
modules.
a. Skin Care - The capability shall be provided for crewmembers to
condition their skin sufficiently to prevent drying and/or cracking.
b. Shaving - Provisions shall be made for crewmembers to shave body
hair.
c. Hair Grooming - Provisions shall be made for crewmembers to cut
hair to maintain the length within mission and/or personal requirements.
d. Nail Care - Provisions shall be made for crew members to trim nails.
e. Body Deodorant - The capability shall be provided for crewmembers
to control body odor.
f. Menstruation - Provisions shall be provided for the collection and
disposal of menstrual discharge.
Refer to Paragraph 10.2.3.4,
Hair Cutting Design Requirements, and
Paragraph 10.2.3.5, Grooming & Shaving Design Requirements,
for facility design requirements.)
7.2.5.3.2 Partial Body Cleansing Design Requirements
{A}
The capability shall be provided for crewmembers to perform selected
body area cleansing as needed.
(Refer to Paragraph 10.2.3.1,
Partial Body Cleansing Design Requirements, for facility design requirements.)
7.2.5.3.3 Oral Hygiene Design Requirements
{A}
The capability shall be provided for crewmembers to maintain proper
oral hygiene. Proper oral hygiene includes tooth, mouth, and gum care.
Water for oral hygiene shall meet potable water quality standards defined
in Paragraph 7.2.2.3.2.
(Refer to Paragraph 10.2.3.3,
Oral Hygiene Design Requirements, for facility design requirements.)
7.2.5.3.4 Whole Body Cleansing Design Requirements
{A}
The capability shall be provided for crewmembers to perform whole body
skin and hair cleansing.
(Refer to Paragraph 10.2.3.2,
Whole Body Cleansing Design Requirements, for facility design requirements.)
7.2.5.3.5 Personal Clothing & Equipment Cleansing Design Requirements
{A}
The capability shall be provided to supply each crewmember with clean
clothing and other washable items, including bedding and linens, over
the duration of the mission.
(Refer to Paragraph 11.13.1.3,
Clothing Design Requirements, and Paragraph
10.10.3, Laundry Facility - Design Requirements, for additional
requirements.)
7.2.5.3.6 Personal
Hygiene Water Design Requirements
{A}
Personal hygiene water is water that is used for external body cleansing.
Personal hygiene water requirements are listed below:
a. Quality - Minimum personal hygiene water quality requirements are
given in Figure 7.2.5.3.6-1.
b. Quantity - Personal hygiene water quantity requirements are given
in Figure 7.2.5.3.6-2. This figure
does not include requirements for laundry and dishwashing which are
system dependent.
c. Temperature - Temperature shall be adjustable from 21 ± 4
°C (70 ± 10 °F) to a maximum of 45°C (113°F).
Figure
7.2.5.3.6-1 Hygiene Water Quality Requirements (Maximum Contaminant
Levels)
| QUALITY PARAMETERS
|
LIMITS |
| PHYSICAL PARAMETERS |
|
| Total solids (mg/l) |
500 |
| Color True (Pt/Co units) |
15 |
| Taste (TTN) |
N/A |
| Odor (TON) |
3 |
| Particulates (max size - microns) |
40 |
| pH |
5.0-8.5 |
| Turbidity (NTU) |
1 |
| Dissolved Gas (free @ 37°C) |
N/A |
| Free Gas (@ STP) |
Note 1 |
| INORGANIC CONSTITUENTS (mg/l) |
|
| (See Notes 2 and 5) |
|
| Ammonia |
0.5 |
| Arsenic |
0.01 |
| Barium |
1.0 |
| Cadmium |
0.005 |
| Calcium |
30 |
| Chlorine (Total - Includes Chloride) |
200 |
| Chromium |
0.05 |
| Copper |
1.0 |
| Iodine (Total - Includes Organic Iodine) |
15 |
| Iron |
0.3 |
| Lead |
0.05 |
| Magnesium |
50 |
| Manganese |
0.05 |
| Mercury |
0.002 |
| Nickel |
0.05 |
| Nitrate (NO3-N) |
10 |
| Potassium |
340 |
| Selenium |
0.01 |
| Silver |
0.05 |
| Sulfate |
250 |
| Sulfide |
0.05 |
| Zinc |
5.0 |
| BACTERICIDE (mg/l) |
|
| Residual Iodine (minimum) |
0.5 |
| Residual Iodine (maximum) |
6.0 |
| AESTHETICS (mg/l) |
|
| Cations |
N/A |
| Anions |
N/A |
| CO2 |
N/A |
Hygiene Water Quality Requirements (Maximum Contaminant Levels)
| QUALITY PARAMETERS
|
LIMITS |
| MICROBIAL |
|
| Bacteria (CFU/100 ml) |
|
| Total Count |
1 |
| Anaerobes |
1 |
| Coliform |
1 |
| Virus (PFU/100 ml) |
1 |
| Yeast & Mold (CFU/100 ml) |
1 |
| RADIOACTIVE CONSTITUENTS (pCi/l) |
Note 3 |
| ORGANIC PARAMETERS (mg/l) |
See Note 2 |
| Total Acids |
500 |
| Cyanide |
200 |
| Halogenated Hydrocarbons |
10 |
| Total Phenols |
1 |
| Total Alcohols |
500 |
| Total Organic Carbon (TOC) |
10,000 |
| Uncharacterized TOC (UTOC) (mg/l) |
1,000
see Note 4 |
| ORGANIC CONSTITUENTS (mg/l) |
See Notes 2 & 5 |
| Notes:
1. No detectable gas using a volumetric gas vs. fluid measurement
system. Excludes CO2 used for aesthetic purposes.
2. Each parameter/constituent MCL must be considered individually
and independently of others.
3. The maximum contaminant level for radioactive constituents
in potable and personal hygiene water shall conform to Nuclear
Regulatory Commission (NRC) regulations (10CFR20, et al.). These
maximum contaminant levels are listed in the Federal Register,
Vol. 51, No. 6, 1986, Appendix B, as Table 2 (Reference Level
Concentrations) Column 2 (Water). Control/containment/monitoring
of radioactive constituents used on SSF shall be the responsibility
of the user. Prior to the introduction of any radioactive constituents
on SSF, approval shall be obtained from the Radiation Constraints
Panel (RCP). The RCP will approve or disapprove proposed monitoring
and decontamination procedures on a case-by-case basis.
4. UTOC equals TOC minus the sum of analyzed organic constituents,
expressed in equivalent TOC.
5. In the event a quality parameter not listed in this table
is projected, or found, to be present in the reclaimed water,
the Water Quality Manager from Man Systems shall be contacted
for a determination of the MCL for that parameter. |
Reference: 278 NASA-STD-3000
Rev. A With Updates, 412b
Figure
7.2.5.3.6-2 Minimum Personal Hygiene Quantity Requirements
| UNITS |
Operational |
90 Day Degraded 1 |
Emergency 3 |
| lb/ person-day |
51.52 |
16.04 |
12.05 |
| kg/ person-day |
23.4 |
8.18 |
5.45 |
| Notes:
1. Degraded levels meet " fail operational criteria".
2. Based on 12 - lb minimum capacity for hygiene and 25 lb used
in a 90-day chamber test. Includes laundry (27.5 lb/person-day)
and dishwashing (12 lb/person-day) quantities.
3. Based on 12 lb/person-day capacity for hygiene and 4 lb/person-day
for laundry.
4. Based on 12 lb/person-day minimum capacity for hygiene only. |
Reference: 278; NASA-STD-3000
499
7.2.6 Pre/Post-Mission
Health Management
{A}
7.2.6.1 Introduction
{A}
This section specifically addresses the health management of the crewmembers
before and after the mission. The other paragraphs of this section deal
primarily with health management during the mission.
7.2.6.2 Pre/Post-Mission Health Management Design Considerations
{A}
Pre- and post-mission measures can be taken to promote the health of
the crewmembers and to increase the chance of a successful mission.
The measures are:
a. Crewmember Selection - Health criteria should be established to
minimize the chances of illness and injury that would result in a loss
of investment in training or a decrement in mission success. Required
physical and psychological aptitudes and abilities of the crew can be
established through careful analysis of the anticipated tasks to be
performed during the mission.
b. Pre-Mission Health Stabilization - A health stabilization program
that includes monitoring must be in place during the preparatory stages
of the mission. Particularly important are immunization and exposure
protection against those diseases that could become overt during the
mission.
c. Pre-Mission Health Training - The goal of this training is to familiarize
the crewmembers with the objectives and modalities of the health maintenance
system including the methods of monitoring to be implemented.
d. Post-Mission Gravitational Readaptation - Readaptation to a 1-G
environment varies by physiological system as shown in
Figure 7.2.6.2-1. The health care, monitoring, and support for readaptation
must consider these factors.
Figure
7.2.6.2-1 Time Course of Physiological Shifts During Readaptation
to 1-G
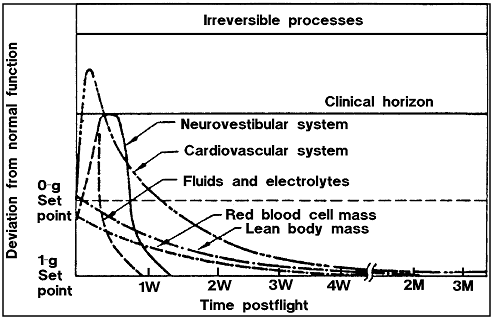
Notes: W - Week, M - Month |
Reference: 208, Fig.
2, p. 135; NASA-STD-3000 192
7.2.6.3 Pre/Post-Mission
Health Management Design Requirements
{A}
The following pre and post-mission health care management programs
shall be provided:
a. Pre-Mission Health Management - Crew selection and training, and
health stabilization programs shall be conducted to:
1. Minimize the possibility of a health problem that would keep a trained
crewmember from going on a mission.
2. Minimize the threat of mission decrement due to a health problem.
b. Post-Mission Health Management - Post-mission health care shall
be provided to minimize the chance of illness or injury to the crewmember
due to his or her deconditioned state.
7.2.7 Health Monitoring
{A}
7.2.7.1 Introduction
{A}
This section addresses the monitoring measures necessary to evaluate
the health of the crewmembers and the health safety of the space module
environment. This section discusses only monitoring of crew health and
water quality. Other sections of this document monitoring requirements
for the space module environment. These other sections are referenced
in the appropriate paragraphs.
7.2.7.2 Routine Health Monitoring Design Considerations
{A}
7.2.7.2.1 Routine Crew Health Monitoring Design Considerations
{A}
The following are considerations for establishing a crew health monitoring
program:
a. Record Keeping - The results of all crew health monitoring must
be kept in a permanent and easily retrievable format for trend analysis.
There must a simple and rapid way to communicate the data to the ground.
The method for handling, storing, and transmission of crew members medicine
health records must be totally secure.
b. Standards - Standards defining nominal limits of the monitored parameters
and procedures for handling health problems (treatment, consultation,
rescue, mission abort, etc.) must be clearly defined and available to
responsible space module crewmembers.
c. Frequency of Monitoring - There should be an increased frequency
of health monitoring at the beginning and the end of the mission compared
with a baseline frequency during the middle of the mission. This is
due to the effect of environmental changes on both the space module
and the crew.
7.2.7.2.2 Water Quality Monitoring Design Considerations
{A}
The water supply characteristics, mission duration, anticipated hardware
lifetime, and rescue opportunities for long-term space missions are
unlike those encountered in any terrestrial application or previous
manned space program. These characteristics introduce a variety of hazards
which dictate the need for unique water quality requirements, monitoring,
capabilities for quality maintenance and restoration, and novel technologies
to enable these activities.
7.2.7.2.2.1 Toxicological Monitoring Design Considerations
{A}
The following are considerations for toxicological monitoring of the
water supply:
a. Water Recycling - To avoid the severe launch or resupply penalties
associated with ground-supplied water, long missions will incorporate
a system to recycle water. Sources for the reclaimed water include cabin
humidity condensate, spent wash water, and urine. Crewmembers will be
exposed to reclaimed water in metabolic, personal hygiene, and housekeeping
activities. Of particular impact will be the water-soluble volatile
and nonvolatile contaminants from the waste, humidity, and condensate
collection systems.
b. Conventional Systems - The maximum allowable concentration limits
for many inorganic chemicals in potable water are below those which
could be detected by conventional process control and screening analyses,
such as conductivity and pH measurements.
c. Chronic Exposure Considerations - Water recycling introduces the
potential for repeated exposure to metabolically active contaminants
and increases the potential in reclamation and disinfection processes
for chemical derivatization of innocuous constituents into toxic products,
such as organohalides. Long missions and continuous habitation will
necessitate that the effects of chronic exposure be considered along
with acute toxicity.
d. Prediction of Toxins - Because toxicant incidence and abundance
vary among different wastewater reclamation techniques, and since the
composition of the source wastewater is highly variable, it is difficult
to define the composition of product water. Of particular concern is
the organic content of the reclaimed water.
e. Total Organic Carbon - Because of the variety and variability of
organics reclaimed water, Total Organic Carbon (TOC) will be a critical
surrogate measurement required for potability verification, process
control, and hygiene quality determination.
f. Exposure Limits - The establishment of exposure limits for the wide
variety of organics found in reclaimed water is a problem of enormous
magnitude. Exposure limits, for the most part, do not exist for organics
that have been identified in reclaimed water because these chemicals
do not correspond to those (such as pesticides, petroleum products,
industrial wastes, and urban and agricultural runoff) encountered in
terrestrial water.
g. System Breakdown - The long system life inherent in long-term missions
increases the potential for accumulation of toxic contaminants, for
system failures and malfunctions, and for contributions to the overall
contaminant burden by degradation of system materials.
h. Stainless Steel - Although stainless steel was successfully used
to fabricate the STS water system, long-duration mission hardware life
requirements may preclude its use.
i. Impact of Experiments and Process - Biological and industrial experiments
and processes to be conducted on long-duration space missions constitute
another potential and undefined source of contamination for the water
system and increase the variability of the source water.
7.2.7.2.2.2 Microbiological Monitoring Design Considerations
{A}
Even in high quality water supplies protected by a residual bactericide,
viable organisms can still persist. Therefore, the potential for microbial
overgrowth is an ever-present hazard. The following are considerations
for microbiological monitoring:
a. Water Recycling - In reclaimed systems, the potential exists for
introduction of microorganisms into the system in greater numbers and
variety than in conventional or previously used water systems.
b. Use of Coliforms - Coliforms, the conventional indicator organism
group for terrestrial potable water, is not an adequate indicator of
total microbial acceptability for aerospace water systems.
c. Disinfectant-Resistant Forms - Recycling of water introduces the
potential for circulating pathogenic or opportunistic organisms, and
increases the potential for selection of disinfectant-resistant microbiological
species.
d. Previous Systems - Previous spacecraft water systems have been required
to maintain throughout the potable water system and have successfully
met this requirement.
e. Time Considerations - If any viable organisms are detected, one
is immediately faced with the task of identification to assess the potential
impact of the particular species. Since medical requirements preclude
crew use of space module water until its quality has been verified,
routine identification would impose additional delays before reclaimed
water could be used.
f. System Maintenance/Reliability - Quality maintenance of the long-term
mission water systems will require careful materials selection to preclude
adverse deterioration over the expected operation of the system, the
maintenance of a continuous residual bactericide downstream of the reclamation
process(es), and a method of restoring system integrity in the event
of system malfunction or contamination.
g. Biofilm Potential - The projected long term use of the water distribution
system will favor the development of biofilms within the system. These
films can harbor organisms and protect them from the residual bactericide.
The resulting microbial contamination or microbial growth products in
the water must be prevented.
7.2.7.2.2.3 Physical Monitoring Design Considerations
{A}
A variety of physical properties are readily measured in conventional
laboratories to determine the quality of water supplies. These parameters
include properties which have direct effects on water acceptability
and those which are indicative of other undesirable conditions. The
following are considerations for physical monitoring of the water supply:
a. Color - consumer rejection because of its effect on aesthetic quality
and is indicative of contaminants.
b. Taste and Odor - evaluations that rely on the human sensory apparatus.
Acceptability of the taste of potable water is important to both the
psychological well-being and the physiological health of the crew. Potable
water provided on previous U.S. manned space flights has been characterized
as tasteless and undesirable. The flat taste of this water is probably
a direct result of its high quality analogous to triple distilled water.
In order to meet maximum concentration limits of potential toxicants,
reclaimed water will have a similar tasteless quality unless additives
are provided to enhance flavor. At times when bactericide overdosage
has occurred, crews have indicated objectionable taste.
c. Turbidity - Turbidity is an indicator of particulate contamination
which may be living or nonliving material. Excessive nonliving particulate
material interferes with disinfection and can cause consumer rejection
for aesthetic reasons. Large particles can harbor microorganisms in
their interior.
d. Other Physical Parameters - Temperature, conductivity, and pH are
other collective physical parameters which affect acceptability of water.
These physical properties may be quite easy to measure and provide rapid,
on-line information about the quality of the water.
7.2.7.3 Routine Health Monitoring Design Requirements
{A}
7.2.7.3.1 Routine Crew Health Monitoring Design Requirements
{A}
The space module shall have the following routine crew health monitoring
capabilities:
a. Routine Diagnostic Physical Examination - The capability for conducting
routine diagnostic physical examination of the crewmembers shall be
provided on all long-term missions (in excess of two weeks).
(Refer to Paragraph 10.9.3.2.14
Physician's Equipment, for equipment required for routine physical examination.)
b. Monitoring During Exercise - Requirements for physiological monitoring
of the crew member during exercise are defined in
Paragraph 7.2.3.3.3, Exercise Countermeasure Design Requirements.
(Refer to Paragraph 10.8.3.2,
Countermeasure Monitoring Design Requirements, for facilities and equipment
for monitoring during exercise.)
c. Pre- and Post-Mission Health Monitoring - Requirements for physiological
monitoring before and after mission are defined in
Paragraph 7.2.6.3, Pre- and Post-Mission Health Management - Design
Requirements.
7.2.7.3.2 Water Quality Monitoring Design Requirements
{A}
The capability to detect, differentiate, and warn the crew as necessary
to maintain crew health for selected contaminants in the space module
water supply by real-time or near-real-time monitoring shall be provided.
The capability to disinfect/sanitize the water system shall be provided.
The following water quality monitoring requirements apply to all space
module water that comes into direct contact with personnel (through
ingestion, personal hygiene, housekeeping, etc.).
7.2.7.3.2.1 Water Quality Monitoring Schedule Design Requirements
{A}
Water quality shall be monitored according to the schedule shown in
Figure 7.2.7.3.2.1-1.
Figure
7.2.7.3.2.1-1 Required Water Quality Monitoring Schedule For All
Water Which Comes Into Contact With Personnel.
| PARAMETER |
ON-LINE1 POT HYG |
OFF-LINE2 POT HYG |
| Physical |
|
|
| Total Solids |
- - |
- - |
| Color |
- - |
+ + |
| Conductivity |
X X |
X X |
| Taste & Odor |
- - |
+ + |
| Particulates |
- - |
+ + |
| pH |
X X |
X X |
| Temperature |
X X |
X X |
| Turbidity |
TBD TBD |
+ + |
| Dissolved Gas |
- - |
+ - |
| Free Gas |
- - |
+ - |
| Inorganics |
|
|
| Ammonia |
- - |
+ + |
| Iodine |
X X |
X X |
| Specific |
|
|
| Inorganics3 |
- - |
+ + |
| Aesthetics |
|
|
| Specific |
|
|
| Contributors4 |
- - |
+ + |
| Microbial |
|
|
| Bacteria |
|
|
| Total Count |
- - |
X X |
| Anaerobes |
- - |
+ + |
| Coliform |
- - |
- - |
| Virus |
- - |
- - |
| Yeast & Mold |
- - |
- - |
| Microbe ID5 |
- - |
X X |
| Radionuclides6 |
- - |
X X |
| Organics |
|
|
| TOC |
X7 X7 |
X X |
| Specific |
|
|
| Organics |
- - |
+ + |
| Notes:
X Denotes that monitoring is required.
- Denotes that monitoring is not required.
+ Denotes that this monitoring requirement will be waived if
verification testing and analysis indicate that the respective
quality parameter limit will be reliably met.
1 Analysis of these process stream samples will be performed
to provide real time or near real time results for process control
and presumptive water quality assessment. Requirements for on-line
monitoring of additional parameters will be established if verification
testing and analysis indicates that such monitoring is required
for process control or water quality assessment.
2 Product water samples from the water systems will be analyzed
off-line for confirmation of water quality. The continued operation
of the ECLSS and the use of the water will not necessarily be
contingent upon the availability of the analyses once the water
systems are verified as being operational. In addition to the
on-line and off-line analyses, grab samples from the water systems
will be obtained for ground, post-mission analysis.
3 Specification of organic and inorganic elements and compounds
to be monitored will be based on the potential for their being
present in the product water and their toxicity. In the event
a parameter not listed in this table is projected, or found, to
be present in the reclaimed water, the Water Quality Manager,
JSC will be contacted for a determination of the monitoring requirements.
4 Selection will be based on determination of critical aesthetic
parameters.
5 This does not include identification of viruses.
6 Inflight monitoring capability will be provided by the specific
experiment or procedure utilizing radionuclides.
7 Analytical procedure may provide an indirect equivalent of
classical TOC. |
NASA-STD-3000 417b, Rev. B
7.2.7.3.2.2 Chemical Monitoring Design Requirements
{A}
The following requirements apply to monitoring of chemical contaminants
in water:
a. Definition of Contaminants - A capability to monitor chemical contaminants
in the space module reclaimed water shall be provided. Requirements
for water quality monitoring of chemical contaminants are included in
Figure 7.2.7.3.2.1-1.
b. Direct measurement - When necessary, organics and inorganics shall
be monitored directly (not through a surrogate).
c. When required, exposure limits shall be established for organics
and inorganics on an individual basis.
7.2.7.3.2.3 Microbiological Monitoring & Treatment Design Requirements
{A}
The following requirements apply to monitoring and treatment of microbiological
qualities of water:
a. Determination of Potability - Capability shall be provided to support
real-time decisions on water potability if organisms are detected.
b. Sampling Technique - Water sampling techniques shall preclude contamination
by the operator during sampling.
c. Iodine - Iodine shall be used as the primary agent to maintain water
microbiological quality
d. Alternative Microbial Control - On long-term missions when there
is a potential for development of organisms resistant to iodine, an
alternative microbial control technique shall be provided.
e. Recovery from Microbial Overgrowth - Provisions shall be made to
recover potable and hygiene water microbial control in the event of
overgrowth using processes that will not degrade the quality of water
with respect to other parameters.
7.2.7.3.2.4 Physical Monitoring Design Requirements
{A}
Equipment shall be provided to meet the physical and aesthetic water
quality monitoring requirements identified in
Figure 7.2.7.3.2.1-1.
7.2.7.3.3 Environmental Monitoring Design Requirements
{A}
Environmental monitoring necessary to maintain crew health shall be
provided as follows:
a. Particulate Monitoring - The capability to detect, differentiate,
and warn the crew as necessary to maintain crew health for selected
particulate contaminants in the space module by real-time or near-real-time
monitoring shall be provided.
b. Microbial Contaminants Monitoring - The capability to monitor, detect,
identify, quantitate, and warn the crew as necessary to maintain crew
health for selected microbial contaminants in the space module by real-time
or near-real-time monitoring, including selected internal surfaces,
shall be provided.
(Refer to Paragraph 5.7.2.2.3,
Ionizing Radiation Monitoring and Dosimetry Design Requirements, for
ionizing radiation monitoring. Refer to Paragraph
5.7.3.2.2, Non-Ionizing Radiation Protection Design requirements,
for non-ionizing radiation monitoring requirements.)
c. Chemical Contaminants Monitoring - The capability to detect, differentiate,
and warn the crew as necessary to maintain crew health for specific
chemical contaminants in the space module by real-time or near-real-time
monitoring shall be provided.
d. Ionizing Radiation Monitoring - Refer to
Paragraph 5.7.2.2.3, Ionizing Radiation Monitoring and Dosimetry
Design Requirements for ionizing radiation monitoring.
e. Non-ionizing Radiation Monitoring - Refer to Paragraph
5.7.3.2.2, Non-ionizing Radiation Protection Design Requirements
for non-ionizing radiation monitoring requirements.
f. Atmospheric Monitoring - Refer to
Paragraph 5.1.3.4, Atmosphere Monitoring and Control Design Requirements,
for atmosphere monitoring requirements.
The capability to decontaminate contaminated areas shall be provided.
(See paragraph 5.1.3)
7.2.8 Biological Payloads
{A}
Biological payloads must meet the specific pathogen-free criteria as
defined by the Human Research Policy and Procedures for Space Flight
Investigations HRPPC) guidelines, JSC 20483. Basic environmental design
requirements and acceptability limits shall minimize infectious agents,
conditions, and cross contamination between the crew and biological
payloads (animals, plants, etc.) that may impact crew health and mission
requirements.
7.3
MEDICAL CARE
{A}
7.3.1 Introduction
{A}
This section presents the minimum functional requirements for a space
medical care facility.
(The equipment and facilities for implementing these medical care requirements
are discussed in Section
10.9, Space Medical Facility.)
7.3.2 Medical Care Design Considerations
{A}
7.3.2.1 Objectives of Medical Care Design Considerations
{A}
A space medical care facility should meet the following objectives:
a. Ensure health and safety (ensure crew safety and optimal health
during routine operations).
b. Prevent excess mortality and morbidity.
c. Prevent mission termination (prevent early mission termination due
to medical contingency).
d. Prevent an unnecessary rescue (provided rescue is a possibility).
e. Increase the probability of success of a necessary rescue (provided
rescue is a possibility).
7.3.2.2 Anticipated Illness and Injuries Design Considerations
{A}
The exact nature of the required medical care depends on the space
mission (the mission duration and goal) and the illness and injuries
that are expected to occur in that mission. The characteristics of the
illness and injuries that are particularly important for design consideration
are listed below:
a. Probability of Disease or Injury Occurrence - This can be determined
through historical data and analysis of the nature of the mission tasks
(some tasks are more likely to cause specific injuries). It must be
remembered that selection and pre-mission monitoring can screen out
many potential illness.
b. Time for a Disease to Become Overt - If a disease has a long incubation
or development period relative to the space mission, then diagnosis
and treatment during the mission becomes less important. However, should
the disease have a short incubation, then diagnosis and treatment become
very important. Therefore, it is imperative that means be available
to determine the presence of these diseases and treat them.
c. Disability Level of the Ill or Injured Crewmember - Should a crewmember
become ill or injured, the seriousness of the illness or injury must
be determined in order to make adjustments in workload schedules, etc.
The space module must have effective diagnostic and preventive measures
against diseases and injuries that would seriously disable the crew
member.
d. Recuperation Period - The medical facility must have effective diagnostic
and preventive measures against diseases and injuries that would disable
the crewmember for an extended period. Should a crewmember require a
lengthy recuperation period, particularly if isolation is necessary,
mission planning would require modification to accommodate the situation.
In addition, provision for recuperation in the medical facility must
be provided.
e. Probable Results of Partial or No Treatment - The medical facility
must primarily be prepared to handle those illness and injuries which
would heal more rapidly with treatment or which would become serious
without treatment.
7.3.2.3 Earth - versus Space-Based Medical Care Design Considerations
{A}
The administration of medical care requires a combination of ground
support and the skills and facilities of the crewmembers. Longer space
missions and longer rescue delays require more reliance on the skills
and resources of the crewmembers themselves. The following are considerations
which effect the medical facility design and the training of the crew:
a. Earth-Based Medical Care - Past space missions have been monitored
continuously by an Earth-based Flight Control Team, which includes medical
personnel as team members. With this system, the medical team obtains
health-related information via spacecraft telemetry. This is supplemented
through use of a private medical conference, as necessary, with the
crew. The information obtained during monitoring is intended to deal
with direct medical problems and also to evaluate circumstances that
appear to be leading toward such problems. This information includes
data concerning status of environmental control systems, radiation exposure,
food supply, water condition, and personal hygiene.
b. Space-Based Medical Care - Medical support of future space operations
will require new philosophies and new technologies. The epicenter of
medical care will shift from ground-based Mission Control Centers to
a space-based medical unit. The minimum projected time for arrival of
a rescue vehicle is mission dependent; in fact, rescue may be unavailable
altogether (such as on a Mars mission). In addition to the delay factor,
there also is the issue of establishing medical criteria for committing
a patient to entry into a 1-G environment, following extended exposure
to microgravity, without endangering his or her condition. These considerations
mean that future space modules must have the personnel, facilities,
and technologies to provide adequate medical care and health maintenance
services, including provision for such microgravity or partial gravity
countermeasures as specially tailored exercise programs.
c. Human Engineering of Medical Facility - Proper human engineering
of the space medical facility can increase the effectiveness of the
medical system and decrease the requirement for extensive crew training.
Information in the remainder of this document (particularly
Section 10.9 Space Medical Facility, and
Section 9.0, Workstations) should be used as the basis for the design
of all medical facilities.
7.3.3 Medical Care Design Requirements
{A}
7.3.3.1 General Design Requirements
{A}
A space module shall have a medical facility which can effectively
provide preventive, diagnostic, and therapeutic medical capabilities
in accordance with U.S. clinically acceptable current and anticipated
medical practice standards.
(Refer to Section 10.9,
Space Medical Facility, for information about the design of the medical
facility.)
7.3.3.2 Prevention Design Requirements
{A}
The space medical facility shall be capable of supporting the administration
of preventive medical care as defined in
Paragraph 7.2, Preventive Care.
7.3.3.3 Diagnostic System Design Requirements
{A}
The space medical facility shall be capable of supporting diagnosis
of anticipated illness and injuries, assessment of their degree and
severity, and the tracking of the overall health status of ill or injured
crewmembers.
7.3.3.4 Treatment (Therapeutics) Design Requirements
{A}
The space medical facility shall be capable of supporting various therapeutic
measures:
a. Treatment - The capability shall exist to treat a crewmember for
anticipated diseases and injuries.
b. Stabilization - The capability shall exist to stabilize a critically
ill crewmember until transportation to an appropriate facility is available.
In the event an illness or injury is not treatable at the module.
c. Handling of Deceased Crewmember - The capability shall exist to
handle a deceased crewmember in an efficient, safe, and acceptable manner.
7.4
CREW SURVIVAL
{A}
7.4.1 Introduction
{A}
7.4.2 Crew Survival Design Considerations
{A}
7.4.3 Crew Survival Design Requirements
{A}
a. The emergency vehicle shall be designed to preclude hazard to the
crew and to allow egress from the crashed vehicle in the event of off
nominal landing loads specified below in
Figure 7.4.3-1.
b. Equipment and attachment structures inside the crew compartment
(including fittings and fasteners) shall be designed for off nominal
landing loads specified below in Figure
7.4.3-1.
Figure 7.4.3-1
Ultimate Inertia Load Factors
NASA-STD-3000 473
7.4.3.1 Medical Kit Design Requirements
{A}
The emergency vehicle shall provide an emergency medical kit listed
in Figure 7.4.3.1-1.
Figure
7.4.3.1-1 Emergency Vehicle Medical Kit
| Airway |
Inventory |
| Oral airway (Min. of 4) |
Min. of 4 |
| Tracheal tube w/atylet (Min. of 2) |
Min. of 2 |
| Larynogoscope (1) |
1 |
| Pertrach Kit (1) |
1 |
| Comox resuscitator (1) |
1 |
| Ambu Bag |
|
| Antiseptics |
|
| Alcohol wipes |
|
| Bandages |
|
| Ace Bandage |
|
| Band-Aids |
|
| Kling |
|
| Sponges |
|
| Telfa pads (4 x 4s) |
|
| Wound pack |
|
| Burns |
|
| Silvadene cream (silver sulfadiazine) |
|
| Decongestants |
|
| Afrin nasal spray (1 bottle) |
1 bottle |
| Diagnostic Equipment |
|
| Blood Pressure cuff |
|
| Stethoscope |
|
| Eye Treatment |
|
| Tearisol eye drops (artificial tears) |
|
| Motion Sickness |
|
| Phenergan, oral |
|
| Scop/Dex |
|
| Pain Medications |
|
| Ascriptin (aspirin) |
|
| Tylenol (acetaminophen w/codeine) |
|
| Miscellaneous |
|
| Scissors |
|
| Tweezers |
|
| Tape (generic adhesive - medical) |
|
| Steri-Strip skin closure |
|
| Penlight |
|
NASA-STD-3000 472, Rev. B
7.4.3.2 Crew Survival Kit Design Requirements
{A}
The emergency vehicle shall provide survival equipment listed in
Figure 7.4.3.2-1.
Figure
7.4.3.2-1 Emergency Vehicle Proposed 24 Hour survival Kit (Post-landing).
| ITEM |
BOTH |
LAND ONLY |
WATER ONLY
|
| Water ( 2 liter/person) |
2 liter/person |
|
|
| Day/night flare |
2 |
|
|
| Thermal blanket (large) |
2 |
|
|
| Chem Lights |
10 |
|
|
| Strobe light |
1 |
|
|
| Pen gun flares |
1 gun, 14 flares |
|
|
| First aid kit |
1 |
|
|
| PRC-112 radio (kit) |
1 |
|
|
| Signal mirror |
1 |
|
|
| Knife |
1 |
|
|
| Sunscreen |
1 |
|
|
| Compass |
1 |
|
|
| Whistle |
1 |
|
|
| Penlight |
2 |
|
|
| SARSAT Beacon |
1 |
|
|
| Motion sickness pills |
|
|
In first aid kit |
| Sea dye marker |
|
|
4 |
| Life raft |
|
|
crew raft |
| Matches |
|
10 |
|
| Fire starter kit |
|
2 |
|
NASA-STD-3000 471, Rev. B
Return to Volume I Home



